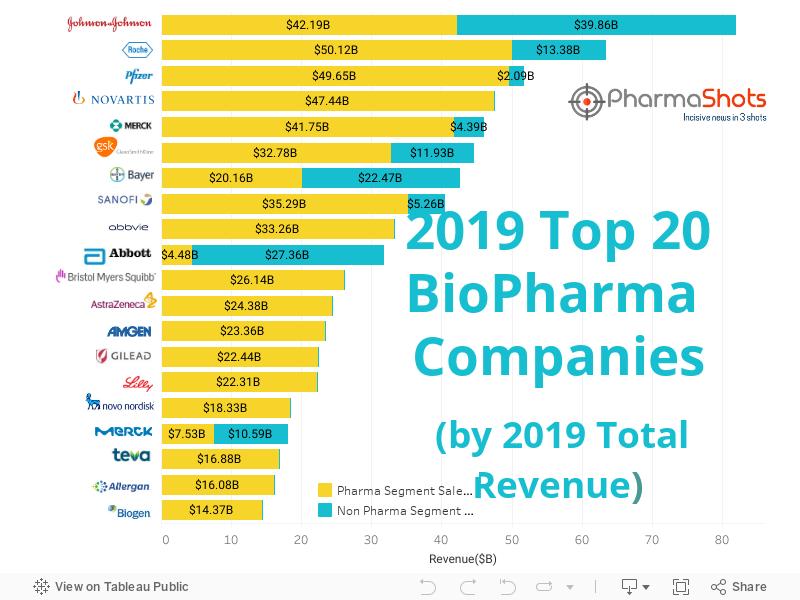
Top 20 BioPharma Companies based on 2019 Total Revenue
- The global biopharma companies are developing novel therapies & devices which is- in turn- enhancing their portfolio and encouraging them in this undergoing competition to stay in the topmost position
- The top 20 companies chartbusters changed their 2019 ranking- but the history of Johnson and Johnson did not change and it continued being at the top again in 2019 as well with a generated revenue of $82.05B
- PharmaShots has compiled a list of top 20 biopharma companies based on their 2019 total revenue
You can also get a PDF version of this report by contacting me through email at connect@pharmashots.com.

Total Revenue: $14.37B Total Employees: ~7-400
Pharma Segment Revenue: $14.37B Non-Pharma Segment Revenue: Nil
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=3907382762&pi=t.aa~a.2937610975~i.33~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745464&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463913&bpp=3&bdt=1034&idt=-M&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0&nras=2&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=1297&biw=1521&bih=730&scr_x=0&scr_y=0&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=0&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=2&uci=a!2&btvi=1&fsb=1&dtd=252
Founded Year: 1978 Headquarter: Massachusetts- United States
Market Cap: ~$55.03B Stock Exchange: NASDAQ
Biogen is a global biopharma company focused on neurology with a broad portfolio including Tecfidera- Avonex- Plegridy- Tysabri- and Fampyra. In 2019- Eisai and Biogen terminated their two P-III clinical trial studies of Elenbecestat (E2609) for Early Alzheimer’s Disease. Additionally- in Mar 2019- Fujifilm acquired Biogen’s Danish biologics manufacturing facility for $890M for the expansion of their bio CDMO business further providing Biogen tax relaxation-.

Total Revenue: $16.08B Total Employees: ~17-400
Pharma Segment Revenue: $16.08B Non-Pharma Segment Revenue: Nil
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=2182344224&pi=t.aa~a.2937610975~i.68~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745464&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463913&bpp=2&bdt=1034&idt=-M&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280&nras=3&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=1989&biw=1521&bih=730&scr_x=0&scr_y=0&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=0&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=3&uci=a!3&btvi=2&fsb=1&dtd=261
Founded Year: 1950 Headquarter: Dublin- Ireland
Market Cap: ~$58.01B Stock Exchange: NYSE
Allergan is an Irish pharma company focused on developing and commercializing pharmaceutical products- biologics- and regenerative medicine around the globe. In Jul 2019- Allergan voluntarily recalled BIOCELL Textured Breast Implants and Tissue Expanders- as a precaution following the US FDA’s notification on global safety of breast implant-associated with anaplastic large cell lymphoma (BIA-ALCL). In Dec 2019- Allergan with its partner Amgen filed a BLA to the US FDA for its ABP 798 (biosimilar- rituximab) which is approved for RA- non-Hodgkin’s lymphoma- chronic lymphocytic leukemia (CLL) in the US and EU.

Total Revenue: $16.88B Total Employees: ~40-039
Pharma Segment Revenue: $16.88B Non-Pharma Segment Revenue: Nil
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=1335245059&pi=t.aa~a.2937610975~i.103~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745464&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463913&bpp=1&bdt=1034&idt=1&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280&nras=4&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=2711&biw=1521&bih=730&scr_x=0&scr_y=0&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=0&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=4&uci=a!4&btvi=3&fsb=1&dtd=270
Founded Year: 1944 Headquarter: Petah Tikva- Israel
Market Cap: ~$9.30B Stock Exchange: NYSE
Teva Pharmaceutical is an Israeli multinational pharma company focused on generics- specialty medicines- and biopharmaceuticals. Teva completed its two-year major restructuring plan in 2019- reducing its cost base by over $3B and net debt by more than $9B. Teva’s worldwide restructuring was started in Dec 2017 leading to the closure or sale of 13 manufacturing sites and shutting down almost 40 offices and labs. In Jul 2019- Teva reported the results of Fremanezumab in P-IIIb FOCUS study to treat migraine in adults. Additionally- in Nov 2019- Teva with its partner Celltrion launched Truxima (biosimilar- Rituxan) in the US.

Total Revenue: $18.13B Total Employees: ~57-071
Pharma Segment Revenue: $7.53B Non-Pharma Segment Revenue: $10.59
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=2881312115&pi=t.aa~a.2937610975~i.138~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745464&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463918&bpp=1&bdt=1039&idt=1&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280%2C852x280&nras=5&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=2873&biw=1521&bih=730&scr_x=0&scr_y=0&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=0&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=5&uci=a!5&btvi=4&fsb=1&dtd=651
Founded Year: 1800 Headquarter: Darmstadt- Germany
Market Cap: ~$12.83 Stock Exchange: ETR
Merck KGaA is a German pharma company that develops therapies in the areas of oncology- neurodegenerative- autoimmune & inflammatory diseases- cardiovascular- fertility- endocrinology- and over-the-counter products. In Mar 2019- Merck KGaA terminated its P-III JAVELIN Ovarian PARP 100 Study with Pfizer targeted for Advanced Ovarian Cancer due to its unmet needs of benefit observed in combination with avelumab. The company also showed some interest in AI by signing an agreement with Iktos for its Artificial Intelligence (AI) Technology in Mar 2019. In Q1’20 the company divested its Allergopharma- its allergen immunotherapy subsidiary to Dermapharm while focusing on the development of therapies for patients with difficult-to-treat diseases.

Total Revenue: $18.33B Total Employees: ~43-258
Pharma Segment Revenue: $18.33B Non-Pharma Segment Revenue: Nil
Founded Year: 1989 Headquarter: Bagsvrd- Denmark
Market Cap: ~$105.16B Stock Exchange: Copenhagen
Novo Nordisk is a global healthcare company focusing on therapies for diabetes- obesity- hemophilia- and growth disorders. In Sep 2019- Novo Nordisk’s Rybelsus (semaglutide) received the US FDA’s approval as the first oral GLP-1 analog for T2D patients. In Nov 2019- the company signed an exclusive license agreement with UBE Industries for its UD-014- which is currently in evaluation for its anti-inflammatory MOA and antioxidative effect on endothelial cells- targeted for NASH.

Total Revenue: $22.31B Total Employees: ~33-625
Pharma Segment Revenue: $22.31B Non-Pharma Segment Revenue: Nil
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=1916794064&pi=t.aa~a.2937610975~i.203~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745490&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463924&bpp=3&bdt=1045&idt=3&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280%2C852x280%2C852x280&nras=6&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=4031&biw=1521&bih=730&scr_x=0&scr_y=1113&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=3&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=6&uci=a!6&btvi=5&fsb=1&dtd=26180
Founded Year: 1901 Headquarter: Indiana- United States
Market Cap: ~$132.49B Stock Exchange: NYSE
Eli Lilly and Company is a global pharmaceutical firm focused on developing therapies in the areas of diabetes- lung cancer- osteoporosis & men’s health. In Aug 2019- – Eli Lilly signed a license agreement with Innovent to develop and commercialize OXM3 in China. In Q2’19 Eli Lilly launched its Emgality in the US to treat Cluster headache and is currently evaluated in P-III in Japan. In Q4’19 Eli Lilly’s Baqsimi received the EU’s approval to treat severe hypoglycemia and was launched in the US in late Q3’19.

Total Revenue: $22.44B Total Employees: ~11-800
Pharma Segment Revenue: $22.44B Non-Pharma Segment Revenue: Nil
Founded Year: 1987 Headquarter: California- United States
Market Cap: ~$95.60B Stock Exchange: NASDAQ
Gilead Sciences is a research-based biopharmaceutical company with a broad portfolio of drugs. In Jul 2019- Gilead signed an exclusive worldwide license agreement with Durect for its HIV and Hepatitis B products. In Oct 2019- Gilead received the US FDA’s approval for Descovy (emtricitabine) to treat HIV Pre-Exposure Prophylaxis. Additionally- in Q1’20 Gilead acquired Forty-Seven for $4.9B to strengthen its immune-oncology portfolio with the addition of Forty Seven’s magrolimab- FSI-174 & FSI-189.

Total Revenue: $23.36B Total Employees: ~23-400
Pharma Segment Revenue: $23.26B Non-Pharma Segment Revenue: Nil
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=4282465370&pi=t.aa~a.2937610975~i.273~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745491&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463931&bpp=1&bdt=1052&idt=1&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280&nras=7&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=4855&biw=1521&bih=730&scr_x=0&scr_y=1951&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=3&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=7&uci=a!7&btvi=6&fsb=1&dtd=27507
Founded Year: 1980 Headquarter: California- United States
Market Cap: ~$122.96B Stock Exchange: NASDAQ
Amgen is one of the leading biotechnology company developing novel therapies focused on cardiology- oncology- neurology- nephrology- and inflammatory diseases. In Oct 2019- the company received Health Canada approval for EVENITY (romosozumab) to treat osteoporosis in postmenopausal women at high risk for fracture. In Nov 2019- Amgen acquired stakes in BeiGene for the expansion of its Oncology Portfolio in China.

Total Revenue: $24.38B Total Employees: ~50-000
Pharma Segment Revenue: $24.38B Non-Pharma Segment Revenue: Nil
Founded Year: 1999 Headquarter: Cambridge- United Kingdom
Market Cap: ~$102.72B Stock Exchange: NYSE
AstraZeneca is a global- biopharmaceutical company focused on oncology- respiratory- neurology- autoimmune- and other therapy areas. The AstraZeneca’s Oncology product Tagrisso alone generated revenue of $3.189B in 2019. In Aug 2019- AstraZeneca with its Partner Fibrogen received the NMPA’s approval for Roxadustat to treat anemia in non-dialysis-dependent patients with chronic kidney disease in China. In early Q1’20 AstraZeneca divested its hypertension therapies portfolio to Atnahs Pharma for $390M.

Total Revenue: $26.14B Total Employees: ~30-000
Pharma Segment Revenue: $26.14B Non-Pharma Segment Revenue: Nil
Founded Year: 1887 Headquarter: New York- United States
Market Cap: ~$123.06B Stock Exchange: NYSE
Bristol-Myers Squibb (BMS) is an American pharmaceutical company focused on oncology- cardiovascular- immunology- and fibrosis. BMS completed acquisition of Celgene in Nov 2019 after the announcement of it in early 2019. In Jun 2019- BMS signed an agreement to sell its manufacturing facility to Catalent to maintain its footprints in Italy. Additionally- in Dec 2019 the company divested its UPSA Consumer Health Business to Taisho for $1.6B with the focus to develop treatments in serious diseases further strengthening Taisho’s OTC platform.

Total Revenue: $31.90B Total Employees: ~107-000
Pharma Segment Revenue: $4.48B Non-Pharma Segment Revenue: $27.39B
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=2462546536&pi=t.aa~a.2937610975~i.378~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745491&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463937&bpp=1&bdt=1058&idt=1&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280&nras=8&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=6061&biw=1521&bih=730&scr_x=0&scr_y=3152&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=3&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=8&uci=a!8&btvi=7&fsb=1&dtd=27814
Founded Year: 1900 Headquarter: Illinois- United States
Market Cap: ~$139.91B Stock Exchange: NYSE
Abbott Laboratories is an American healthcare company with a broad pipeline of generics- diagnostic- natural products- cardiovascular and neuromodulation products along with medical devices and diagnostics. In early Q2’19- Abbott signed an agreement with Banyan Biomarkers to develop Traumatic Brain Injury (TBI) assessment blood test for patients with traumatic brain injury in the US. In Apr 2020- Abbott launched its third a lab-based serology blood test to detect AB- IgG that identifies a person with COVID-19.

Total Revenue: $33.26B Total Employees: ~30,000
Pharma Segment Revenue: $33.26B Non-Pharma Segment Revenue: Nil
Founded Year: 2012 Headquarter: Illinois, United States
Market Cap: ~$111.10B Stock Exchange: NYSE
AbbVie is a global, research-based biopharmaceutical company that develops therapies majorly for chronic autoimmune diseases, oncology, virology with additional targets like cystic fibrosis and women’s health and has enriched pipeline in immunology and neuroscience. In Q2’19, AbbVie acquired Allergan for $63B for the expansion of its revenue base with the addition of Allergan’s medical aesthetics and ophthalmology portfolio. In Nov 2019, AbbVie initiated its P-III trial to evaluate RINVOQ for assessing the safety and efficacy in adult patients with axial spondylarthritis.
Total Revenue: $40.55B Total Employees: ~100,000
Pharma Revenue: $35.29B Non-Pharma Segment Revenue: $5.26
Founded Year: 1973 Headquarter: Paris, France
Market Cap: ~$111.16B Stock Exchange: EPA
Sanofi is a global healthcare leader in vaccines providing healthcare solutions in 170+ countries around the world. Sanofi is ranked third in the global market and first in EU and Latin America. In Mar 2020, Sanofi signed an agreement with Translate Bio to develop a novel mRNA vaccine against COVID-19. In late 2019, Sanofi acquired Synthorx for $2.5B with the focus to enhance its immuno-oncology portfolio with the addition of Synthorx’s THOR-707. Additionally, in Q1’20 Sanofi collaborated with BARDA to facilitate the development of a vaccine against coronavirus.
Total Revenue: $44.78B Total Employees: ~100,000
Pharma Segment Revenue: $34.11B Non-Pharma Segment Revenue: $11.93B
https://googleads.g.doubleclick.net/pagead/ads?gdpr=0&us_privacy=1—&gpp_sid=-1&client=ca-pub-9265584400550148&output=html&h=280&adk=4067407187&adf=3015869866&pi=t.aa~a.2937610975~i.429~rp.4&w=852&abgtt=6&fwrn=4&fwrnh=100&lmt=1743745492&num_ads=1&rafmt=1&armr=3&sem=mc&pwprc=9424952870&ad_type=text_image&format=852×280&url=https%3A%2F%2Fpharmashots.com%2F7518%2Ftop-20-biopharma-companies-based-on-2019-total-revenue&fwr=0&pra=3&rh=200&rw=852&rpe=1&resp_fmts=3&wgl=1&fa=27&uach=WyJXaW5kb3dzIiwiMTkuMC4wIiwieDg2IiwiIiwiMTM1LjAuNzA0OS40MSIsbnVsbCwwLG51bGwsIjY0IixbWyJHb29nbGUgQ2hyb21lIiwiMTM1LjAuNzA0OS40MSJdLFsiTm90LUEuQnJhbmQiLCI4LjAuMC4wIl0sWyJDaHJvbWl1bSIsIjEzNS4wLjcwNDkuNDEiXV0sMF0.&dt=1743745463947&bpp=2&bdt=1068&idt=2&shv=r20250403&mjsv=m202504010101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D93ad4f2cb6db9efe%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MZS_QdB5YthXTydRGmTP0mS3bTEiQ&gpic=UID%3D0000108832690403%3AT%3D1743660961%3ART%3D1743745327%3AS%3DALNI_MbJwxow-ZjN6Qdm72xJ08gekgt-kQ&eo_id_str=ID%3D5c6e1dd85f610f93%3AT%3D1743660961%3ART%3D1743745327%3AS%3DAA-AfjbEovGr5ivBpgWMB887rJqm&prev_fmts=0x0%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280%2C852x280&nras=9&correlator=307905126508&frm=20&pv=1&u_tz=330&u_his=50&u_h=864&u_w=1536&u_ah=816&u_aw=1536&u_cd=24&u_sd=1.25&dmc=8&adx=334&ady=7693&biw=1521&bih=730&scr_x=0&scr_y=4777&eid=95355973%2C95355975%2C95353386%2C95344788%2C31089209%2C95356787%2C95356929&oid=2&pvsid=4306170822855716&tmod=1700464984&uas=3&nvt=1&ref=https%3A%2F%2Fpharmashots.com%2Fcategory%2Ftop20&fc=1408&brdim=0%2C0%2C0%2C0%2C1536%2C0%2C1536%2C816%2C1536%2C730&vis=1&rsz=%7C%7Cs%7C&abl=NS&fu=128&bc=31&bz=1&td=1&tdf=2&psd=W251bGwsbnVsbCxudWxsLDNd&nt=1&ifi=9&uci=a!9&btvi=8&fsb=1&dtd=28240
Founded Year: 2000 Headquarter: Brentford, United Kingdom
Market Cap: ~$81.19B Stock Exchange: LON
GlaxoSmithKline is a global healthcare company serving the world with drugs, vaccines & consumer healthcare products and is a leader in the areas of respiratory and HIV. In Q4’19, GSK divested its Rabipur and Encepur vaccines to Bavarian Nordic for ~1B and received EC’s approval for Benlysta (belimumab, IV) as the first biologic to treat Lupus in children. In Apr 2020, the company also signed an agreement with Sanofi to develop the adjuvanted COVID-19 vaccine.
Total Revenue: $46.84B Total Employees: ~71,000
Pharma Segment Revenue: $41.75B Non-Pharma Segment Revenue: $4.39B
Founded Year: 1891 Headquarter: New Jersey, United States
Market Cap: ~$195.17B Stock Exchange: NYSE
Merck & Co. is a global healthcare company delivering innovative health care products with its prescription medicines, vaccines, biologic therapies, and animal health care products and the company operates in four major segments of Pharmaceutical, Animal Health, Healthcare Services, and Alliances segments. In 2019, Merck acquired ArQule for $2.7B, with the focus to strengthen its oncology portfolio with the addition of ArQule’s ARQ 531 candidate targeted for hematological malignancies. In Feb 2020, Merck planned to spin-off its women’s health, legacy brands, and biosimilars businesses into a NewCo.
Total Revenue: $47.44B Total Employees: ~109,000 Employees
Pharma Segment Revenue: $47.44B Non-Pharma Segment Revenue: Nil
Founded Year: 1996 Headquarter: Basel, Switzerland
Market Cap: ~$204.76B Stock Exchange: SIX Swiss Exchange, NYSE
Novartis is a multinational group of companies specializing in research, development, manufacturing, and marketing with a broad range of healthcare solutions including generic and ophthalmic therapies. Novartis acquired The Medicines Company in Nov 2019 for $9.7B. In Dec 2019, Novartis terminates its 2017 agreement with Akcea for two antisense therapies AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx for cardiovascular disease. Additionally, in May 2019, Novartis acquired WW rights for Takeda’ Xiidra (lifitegrast ophthalmic solution) for $5.3B to strengthen it’s ophthalmic & front-of-the-eye portfolio.
Total Revenue: $44.88B Total Employees: ~103,824
Pharma Segment Revenue: $20.16B Non-Pharma Segment Revenue: $22.47B
Founded Year: 1863 Headquarter: Leverkusen, Germany
Market Cap: ~$58.53B Stock Exchange: ETR
Bayer is a leading life science firm with three divisions pharmaceuticals, consumer health, and crop science. In 2019, Bayer signed an option, research, and license agreement with Dewpoint’s to develop the therapies for cardiovascular and gynecological diseases. Additionally, in Oct 2019, the company terminated its JV agreement with CRISPR Therapeutics signed in 2015. In early 2020, Bayer partners with Population Health Research Institute (PHRI) on global clinical research to evaluate COVID-19 treatments.
Total Revenue: $51.75B Total Employees: ~83,000
Pharma Segment Revenue: $49.65B Non-Pharma Segment Revenue: $2.09B
Founded Year: 1849 Headquarter: New York, United States
Market Cap: ~$181.24B Stock Exchange: NYSE
Pfizer is a research-based, global biopharmaceutical company with a vast portfolio, including vaccines and health care products. In Q4’19, Pfizer’s biosimilar portfolio roll out with emerging approval as it received EMA’s CHMP positive opinion for Amsparity (biosimilar, adalimumab) to treat RA, JIA, axSpA, PsA, psoriasis, hidradenitis suppurativa, CD, UC, uveitis, and pediatric plaque psoriasis. Also, in Dec 2019, the company signed a global license agreement with Theravance for its Pan-JAK inhibitor program.
Total Revenue: $63.50B Total Employees: ~98,000
Pharma Segment Revenue: $50.12B Non-Pharma Segment Revenue: $13.38B
Founded Year: 1896 Headquarter: Basel, Switzerland
Market Cap: ~$277.29B Stock Exchange: SIX Swiss Exchange
Roche is a Swiss multinational healthcare company operating worldwide under pharmaceuticals and diagnostics divisions. In Nov 2019, Roche acquires Promedior for $1.39B, while the acquisition will leverage Roche’s drug development capabilities and expertise to advance PRM-151 in fibrotic diseases, including idiopathic pulmonary fibrosis and myelofibrosis. In Dec 2019, Roche signed an exclusive license agreement with Sarepta Therapeutics to commercialize SRP-9001 (outside the US), targeted for Duchenne muscular dystrophy (DMD). In Q1’20, Roche’s cobas SARS-CoV-2 Test receives the US FDA’s emergency use authorization to identify coronavirus.
Total Revenue: $82.05B Total Employees: ~132,100
Pharma Segment Revenue: $42.19B Non-Pharma Segment Revenue: $39.86B
Founded Year: 1887 Headquarter: New Jersey, United States
Market Cap: ~$350.66B Stock Exchange: NYSE
Johnson & Johnson (J&J) is an American multinational healthcare company focused on development and commercialization of pharmaceutical, medical device, and consumer packaged products. The consumer department of the company focuses on Baby & Beauty, Health & Healing Products, and the medical device sector is focused on Orthopedics and Cardiovascular Diseases. In Apr 2019 J&J received the US FDA’s approval for Balversa (erdafitinib) to treat LA or mUC with Certain FGFR genetic alteration. Additionally, in July 2019 J&J received CHMP’s positive opinion for expanded use of Imbruvica (ibrutinib) in previously untreated CLL and Waldenström’s macroglobulinemia (WM) in EU. In the early 2020, J&J and BARDA funded ~$1B in R&D of novel COVID-19 vaccines and initiated the development of vaccines against COVID-19.
Sources: Company annual reports, SEC filings, press releases, and company websites
Market Cap source: Google finance (as on 1st Apr 2020)
All revenues are reported in USD.
Note: Takeda is not included in top 20 list, as its actual revenue is not available (2019 forecasted revenue is $19.59B) (Yen to USD conversion rate as on 1st Apr 2020)
Related Post: Top 20 BioPharma Companies based on 2018 Total Revenue
